Unmasking bias in your survival analysis
Competing risks methods are advanced statistical approaches designed to analyze multiple events that may simultaneously impact a patient, using methods such as cumulative incidence functions and Fine & Gray models. These methods are essential in survival analysis, particularly in situations where multiple risks can interact and interfere with another, making traditional survival analyses potentially biased or incomplete.
Survival analysis
Survival analysis encompasses a wide range of statistical methods designed to handle censored data. Censoring occurs when we can only partially observe the timing of an event – we know that a subject hasn’t experienced the event (such as death, system failure, or disease recurrence) up until a certain date, but we lose track of them after that point. These analyses allow for the inclusion of incomplete information in the analysis, enabling researchers to estimate survival probabilities and time-to-event outcomes more accurately.
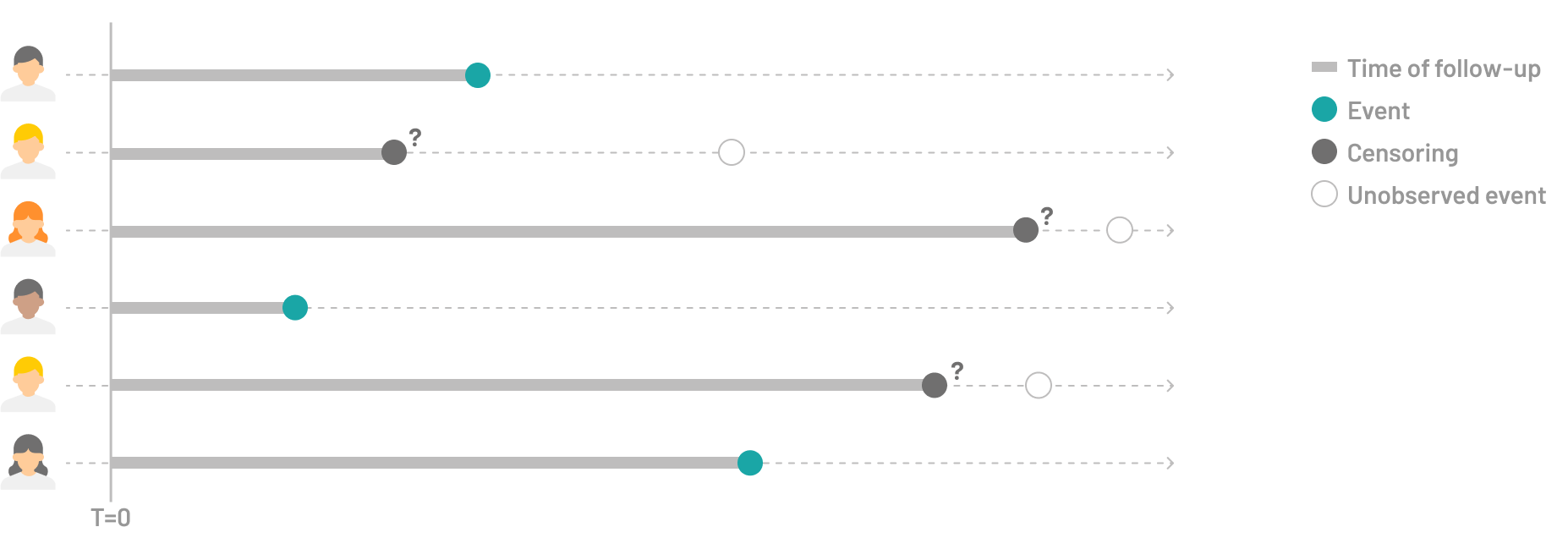
- the presence or absence of the event of interest,
- the time to event for patients having the event of interest or time to censoring for patients without the event of interest.
What is a competing risk?
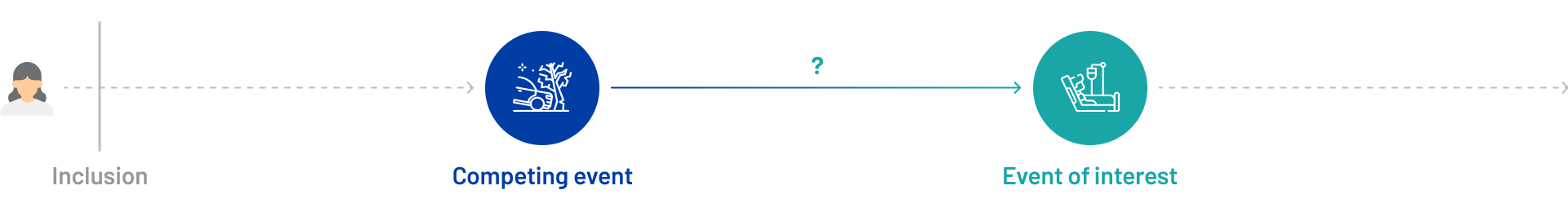
A competing risk is an event that can occur in place of the event of interest, thereby preventing its occurrence or accurate observation. For instance, if a patient dies prior to being hospitalized, the hospitalization event is no longer observable.
How competing risks can bias your analysis and strategy for addressing them
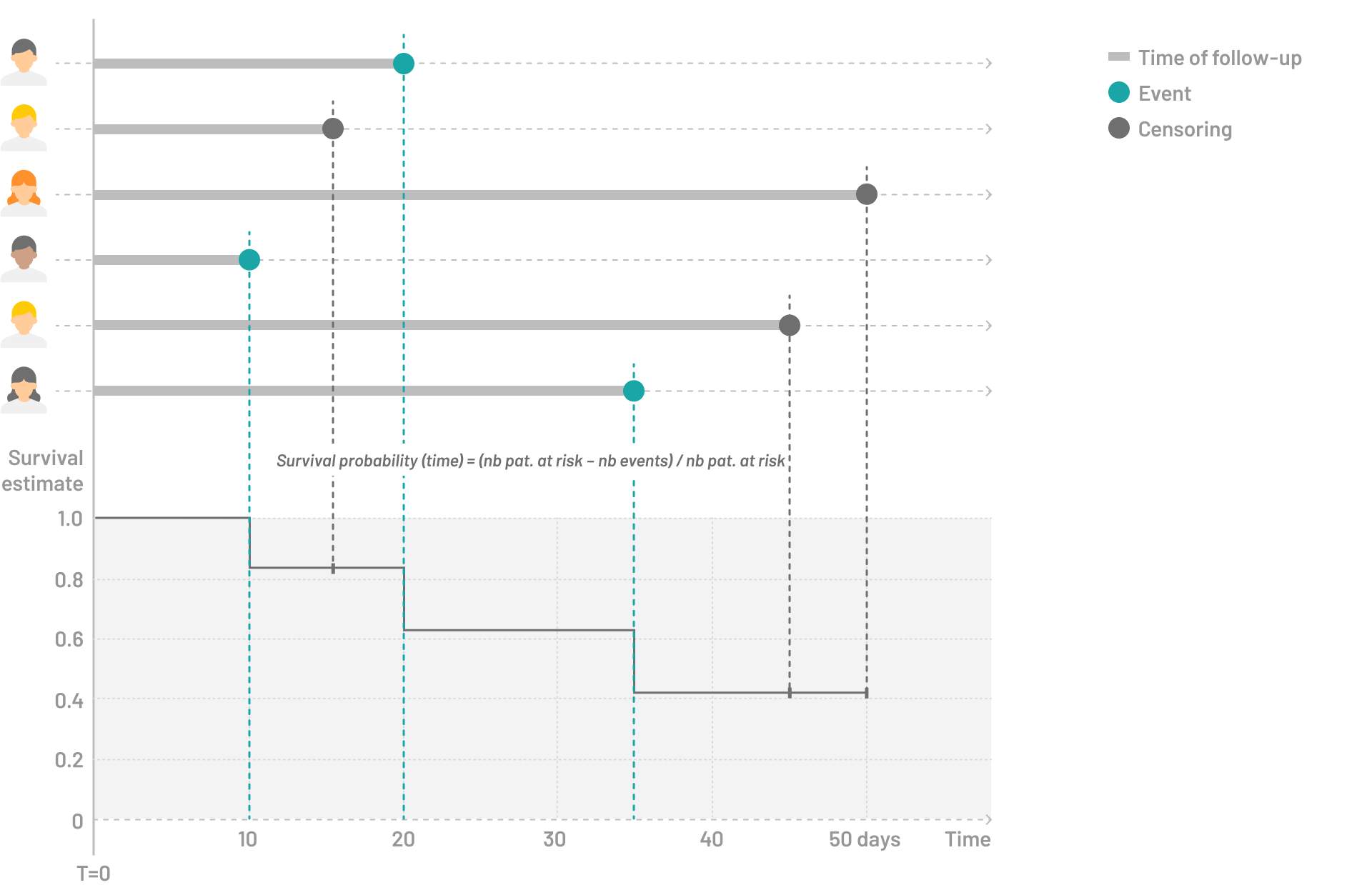
The assumption underlying traditional survival analysis is that censored patients are presumed to have the same risk profile as those who remain under observation. This means that, despite being censored—either due to loss to follow-up, withdrawal, or the end of the study period—these patients are treated as if they would have experienced the event of interest had they continued to be monitored. However, in the context of competing risks, this assumption becomes problematic. If a competing event occurs before the event of interest, it nullifies the risk of the event of interest for that patient, reducing its probability of occurrence to zero. This is because the competing event precludes the possibility of the event of interest happening, a phenomenon that conventional survival analysis methods fail to address.
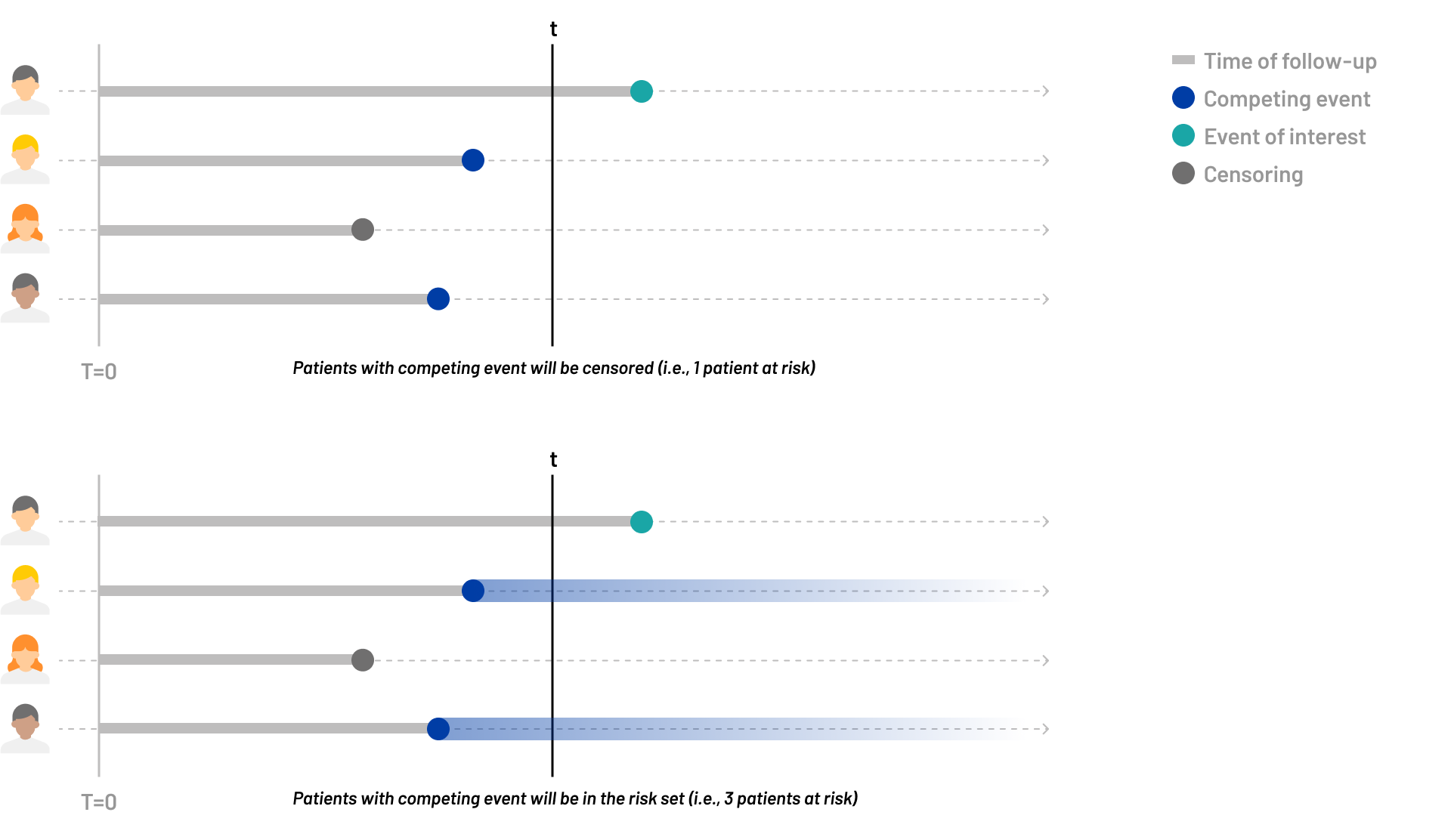
To account for competing events, a virtual risk set should be created that includes patients actually at risk of the event of interest but also patients who have experienced a competing event, effectively reflecting that their risk of having the primary event is zero. While this modified risk set may no longer be directly interpretable, it allows for a direct translation into the cumulative incidence rate of the event of interest.
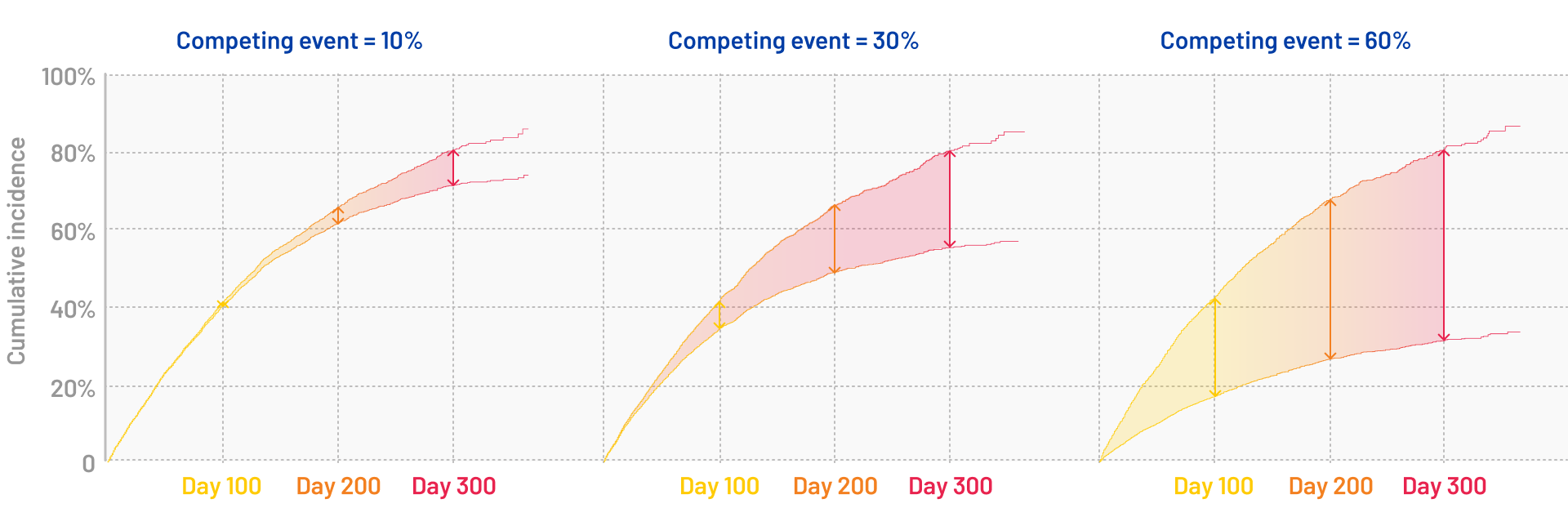
Heva studied the potential impact of neglecting competing events in survival analysis (see the poster). The findings demonstrated that ignoring competing risks leads to systematic bias in results. This bias becomes more pronounced under two key conditions:
- when competing events occur more frequently,
- when the follow-up period extends longer.
Applications of competing risk analyses
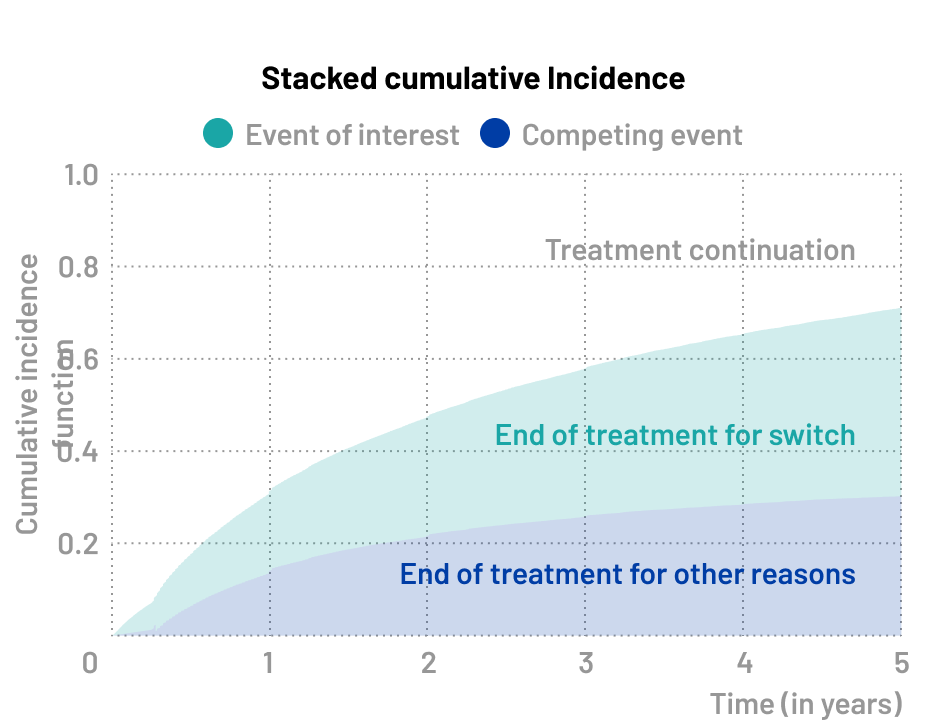
Treatment discontinuation
Treatment discontinuation is a classic competing risks scenario, as patients may stop treatment for various reasons: experiencing adverse effects, failing to adhere to the treatment i.e. non-adherence, dying before treatment completion, or achieving their therapeutic goals (e.g. clinical remission or desired treatment outcome).
❓Research questions
- What is the rate of treatment discontinuation due to death or treatment switch?
- What is the impact of patient characteristics on treatment discontinuation ?
🔍 Event of interest
- Treatment switch
⚠️ Competing event
- Treatment discontinuation for others reasons (death, adverse event)
Clinical events
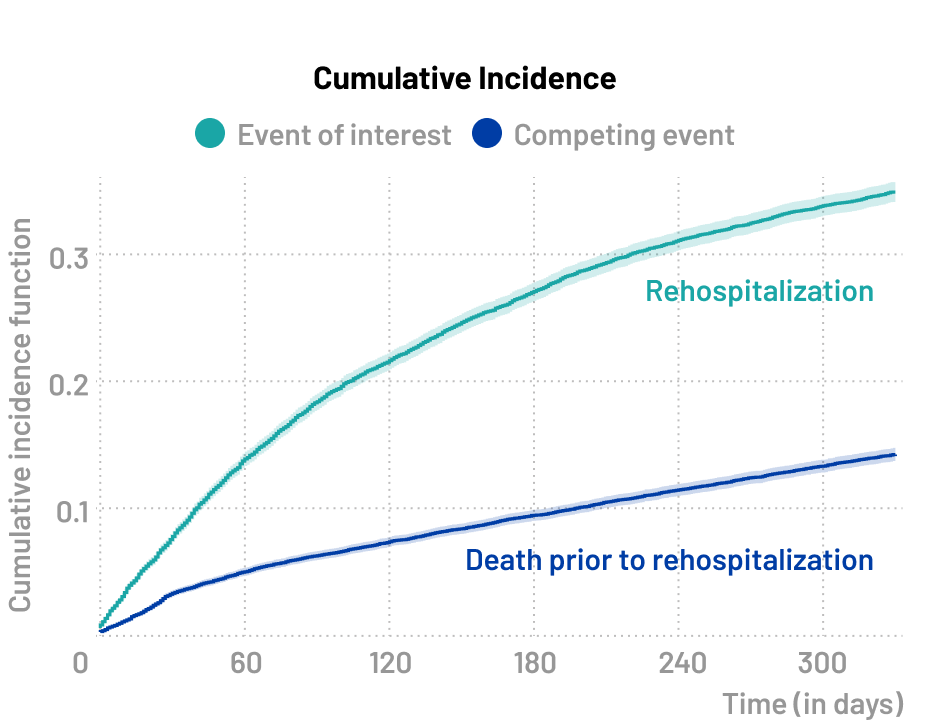
A specific type of competing risk scenario, known as a semi-competing risk situation, arises when the occurrence of death precludes the occurrence of the clinical event of interest, while the reverse is not true. The clinical event of interest could involve the occurrence of adverse events, disease progression, or treatment failure.
❓Research questions
- What is the rate of rehospitalizations following initial surgery?
- What is the impact of the surgical method on rehospitalization rates?
🔍 Event of interest
- Rehospitalization
⚠️ Competing event
- Death prior to rehospitalization
Conclusion