Unmasking bias in your survival analysis
Competing risks methods are advanced statistical approaches designed to analyze multiple events that may simultaneously impact a patient, using methods such as cumulative incidence functions and Fine & Gray models. These methods are essential in survival analysis, particularly in situations where multiple risks can interact and interfere with another, making traditional survival analyses potentially biased or incomplete.
Ce même bloc avec des personnalisation de largeur de colonne (test avec un icône à droite d’un paragraphe)
Competing risks methods are advanced statistical approaches designed to analyze multiple events that may simultaneously impact a patient, using methods such as cumulative incidence functions and Fine & Gray models. These methods are essential in survival analysis, particularly in situations where multiple risks can interact and interfere with another, making traditional survival analyses potentially biased or incomplete.
Survival analysis
Survival analysis encompasses a wide range of statistical methods designed to handle censored data. Censoring occurs when we can only partially observe the timing of an event – we know that a subject hasn’t experienced the event (such as death, system failure, or disease recurrence) up until a certain date, but we lose track of them after that point. These analyses allow for the inclusion of incomplete information in the analysis, enabling researchers to estimate survival probabilities and time-to-event outcomes more accurately.
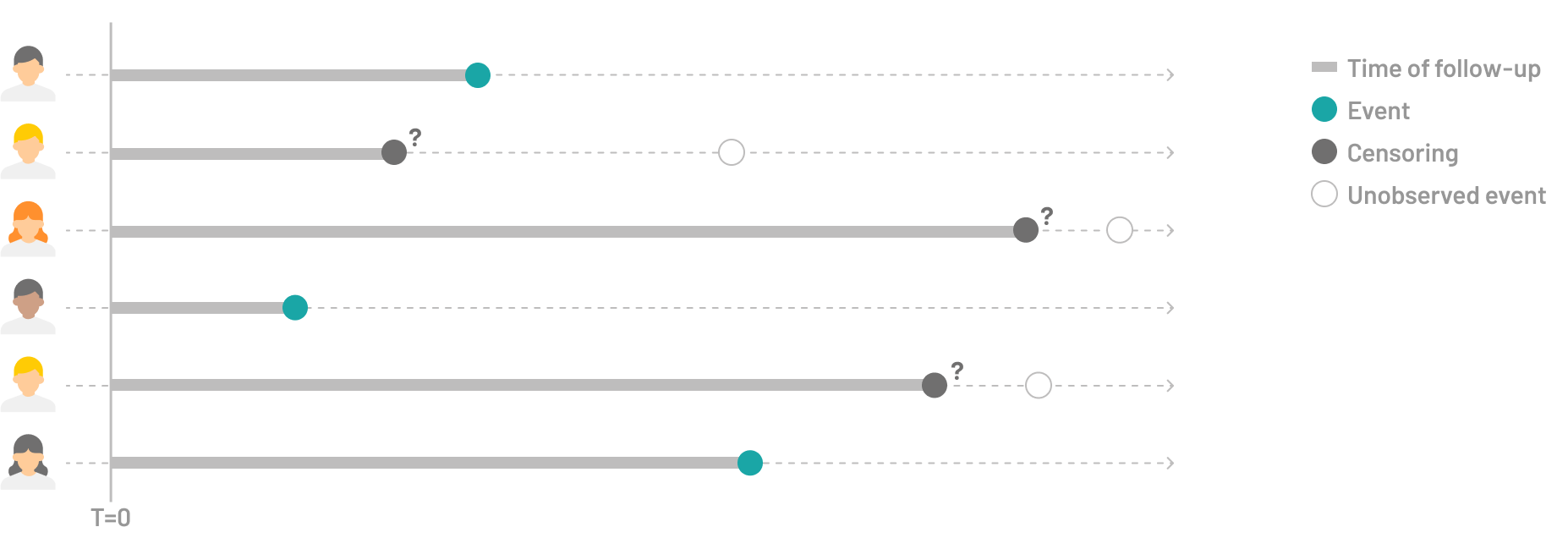
What is a competing risk?
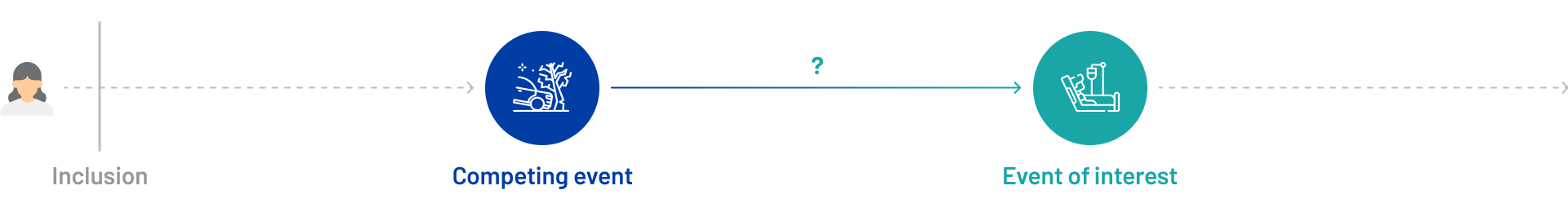
A competing risk is an event that can occur in place of the event of interest, thereby preventing its occurrence or accurate observation. For instance, if a patient dies prior to being hospitalized, the hospitalization event is no longer observable.
Applications of competing risk analyses
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.

Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.

Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.

Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.

Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.

Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.

Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.

Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Your content goes here.
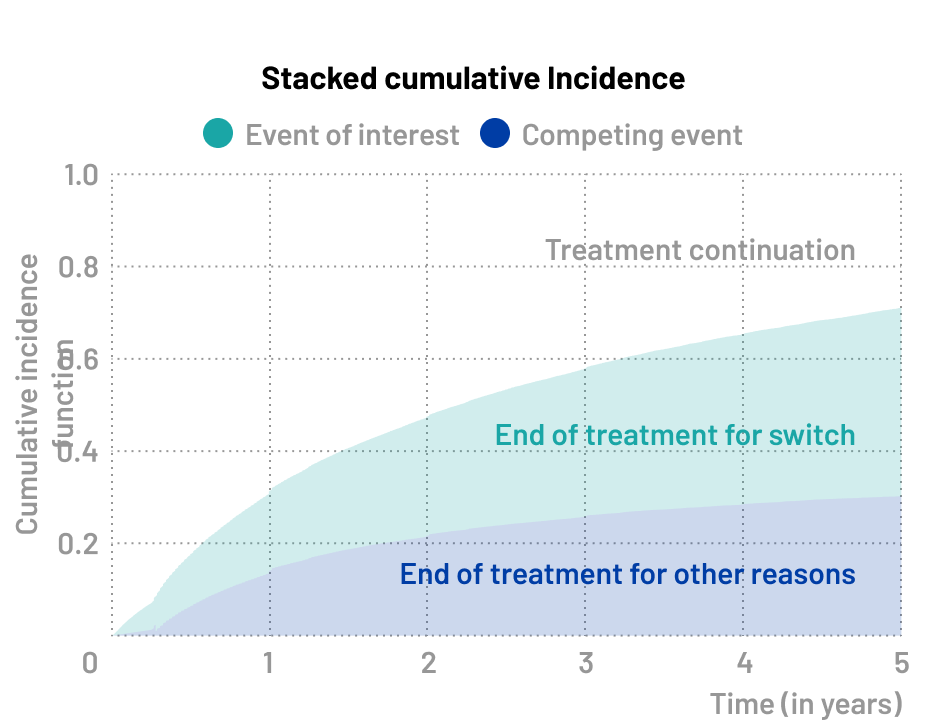
Treatment discontinuation
Treatment discontinuation is a classic competing risks scenario, as patients may stop treatment for various reasons: experiencing adverse effects, failing to adhere to the treatment i.e. non-adherence, dying before treatment completion, or achieving their therapeutic goals (e.g. clinical remission or desired treatment outcome).
❓Research questions
- What is the rate of treatment discontinuation due to death or treatment switch?
- What is the impact of patient characteristics on treatment discontinuation ?
🔍 Event of interest
- Treatment switch
⚠️ Competing event
- Treatment discontinuation for others reasons (death, adverse event)
Clinical events
A specific type of competing risk scenario, known as a semi-competing risk situation, arises when the occurrence of death precludes the occurrence of the clinical event of interest, while the reverse is not true. The clinical event of interest could involve the occurrence of adverse events, disease progression, or treatment failure.
❓Research questions
- What is the rate of rehospitalizations following initial surgery?
- What is the impact of the surgical method on rehospitalization rates?
🔍 Event of interest
- Rehospitalization
⚠️ Competing event
- Death prior to rehospitalization
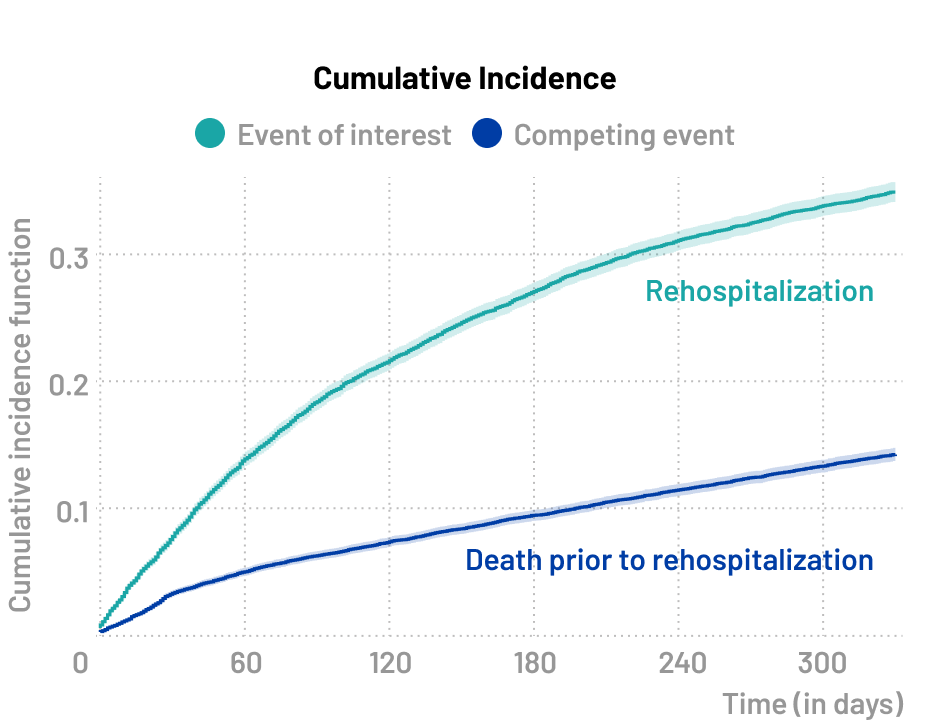
Tableau à styliser
| Données primaires | SNDS | |||
|---|---|---|---|---|
| image ici | Représentativité | Échantillon | Exhaustivité | |
| image ici | Caractéristiques socio-démographiques des patients |
Sur mesure | Âge, sexe, lieu de naissance, lieu de résidence, statut vital |
Insertion de vidéo youtube
| Critère | Données primaires | SNDS | |
|---|---|---|---|
 |
Représentativité | Données primaires Échantillon | SNDS Exhaustivité |
| Caractéristiques socio-démographiques des patients | Données primaires Sur mesure : Âge, sexe, lieu de naissance, lieu de résidence, statut vital | SNDS Âge, sexe, lieu de naissance, lieu de résidence, statut vital | |
 |
Description de la maladie | Données primaires Diagnostic et histoire de la maladie, évaluation clinique (stade / sévérité) | SNDS Diagnostic et histoire de la maladie (proxy) |
| Parcours de prise en charge |
Données primaires
Description des traitements et du parcours de prise en charge Motifs d'arrêt de traitement, tolérance / sécurité |
SNDS Traitements remboursés, parcours thérapeutiques | |
 |
Paramètres biologiques et cliniques | Données primaires Résultats examens biologiques radiologiques / investigations cliniques | SNDS - |
 |
Qualité de vie | Données primaires PROMs / PREMs | SNDS - |
 |
Coûts | Données primaires Recueil fastidieux et compliqué avec une qualité discutable | SNDS |
 |
Période d'étude - suivi | Données primaires Suivi à court et long terme, mais étude coûteuse | SNDS Suivi long terme (morbidité, efficacité, statut vital) |
| Critère | ✓ Utilisation du NIR | ✗ Pas d'utilisation du NIR | |
|---|---|---|---|
| Méthodologie d'appariement |
✓ Utilisation du NIR
Appariement direct (Appariement reposant sur le numéro d’identification anonyme du patient : nécessite son recueil dans la base à apparier, date de naissance et sexe) |
✗ Pas d'utilisation du NIR
Appariement indirect (Appariement reposant sur des variables communes aux deux bases et suffisamment discriminantes : date de naissance, sexe, dates de soins…) |
|
 |
Autorisation réglementaire | ✓ Utilisation du NIR Autorisation CNIL | ✗ Pas d'utilisation du NIR Méthodologie de référence |
| Qualité de l'appariement |
✓ Utilisation du NIR
Excellent (proche de 100%) |
✗ Pas d'utilisation du NIR Variable selon la qualité et la typologie de la base | |
| Responsable d'appariement | ✓ Utilisation du NIR Appariement réalisable par la CNAM | ✗ Pas d'utilisation du NIR Appariement réalisable par le bureau d'étude |
 App Store
App Store
|
 Inhouse
Inhouse
|
 Custom Apps
Custom Apps
|
|
|---|---|---|---|
| Type de compte |
 App Store
App Store
(99€/an) |
 Inhouse
Inhouse
(299€/an) |
 Custom Apps
Custom Apps
(99€/an) |
| Accès à l’application |
 App Store
App Store
|
 Inhouse
Inhouse
|
 Custom Apps
Custom Apps
|
| Validation de l’application |
 App Store
App Store
|
 Inhouse
Inhouse
|
 Custom Apps
Custom Apps
|
| Plateforme de distribution et mise à jour |
 App Store
App Store
|
 Inhouse
Inhouse
|
 Custom Apps
Custom Apps
|
| Durée de vie de l’application |
 App Store
App Store
|
 Inhouse
Inhouse
et 1 an |
 Custom Apps
Custom Apps
|
Conclusion